Date Palm
Propagation
1.
Introduction
Although
economically important, palms are a much neglected plant group
in terms of understanding development and propagation potential
thereof. Furthermore, progress in the field of breeding, genetics, crop
improvement, and expansion of commercial plantings for palm has been
restricted by the habit and long-lived nature of these monocotyledonous
trees. Most palms can only be propagated by seeds, i.e., Coconut and
Oil palm.
There are three techniques to propagate date
palm: Seed propagation,
offshoot propagation (traditional methods), and the recently developed
tissue culture techniques. This chapter will highlight each of these
techniques.
2. Seed
propagation
Seed
propagation, also called sexual propagation, although useful for
breeding purposes, is not a proper method of date palm vegetative
propagation, and should be discouraged. Reasons in favour of
discouraging seed propagation, are the following:
* Date palm is a dioecious
species and consequently half of the progeny
will be males and half will be females, with no certain way to
determine at an early stage the sex of the progeny, nor fruit or pollen
quality prior to flowering (often only seven years later);
* Female plants originating from seedlings usually produce late
maturing fruits of variable and generally inferior quality compared to
established clonal palms. In a seedling plantation it is rare that more
than 10 percent of the palms produce fruit of satisfactory quality;
* Date palms are heterozygous, and thus there will be much variation
within the progeny, and desirable characteristics of the parent palm
may be lost. In other words, it is not true to type propagation and no
two seedling palms are alike;
* Seedlings differ considerably with regard to production potential,
fruit quality and harvesting time, making them very difficult to market
as one harvest;
* The above reasons result in waste of time, space and money. |
Thus,
seed propagation is by far the easiest and quickest method of
propagation. However, it is not a true to type propagation technique
and no two seedlings will be alike. Because of its diversity, the seed
approach could only be useful for breeding purposes. When conditions
are known to be unfavourable for date fruit production (case of
marginal areas), the planting of date seeds, for future selection on
fruit quality, is the most economical way of selecting clones that have
some desirable characters such as rain and/or salt tolerance (Figure
34).
Taking the above into consideration, and also
because of the many
reasons listed below, date growers are encouraged to use tissue
culture-derived material of known varieties with high date quality and
marketing potential.
3. Offshoot
propagation
Offshoot
propagation, also called asexual or vegetative propagation,
offers the following advantages:
(i) Offshoot plants are true to
type to the parent palm. The offshoots
develop from axillary buds on the trunk of the mother plant and
consequently the fruit produced will be of the same quality as the
mother palm and ensures uniformity of produce.
(ii) The offshoot plant will bear fruits 2 - 3 years earlier than
seedlings. The life span of the date palm is divided into two distinct
developmental phases: vegetative, in which buds forming in the leaf
axils develop into offshoots; and generative, in which buds form
inflorescences and offshoots cease. From the time that the axillary bud
of a leaf has differentiated into an offshoot until the time it grows
outwards, takes up to three years (18 to 36 months), with another three
to four years before it reaches the desired size for its separation and
planting (Hilgeman, 1954).
|
Offshoots
are mainly produced in a limited number (20 to 30 at most)
during the early life of the palm (10 to 15 years from the date of its
planting) depending on the variety and on prior fertilisation
treatment, irrigation and earthing up around the trunks, (Nixon and
Carpenter, 1978). Although 20 to 30 offshoots are produced by a palm,
only three or four offshoots are suitable for planting out in one year
and must still go into the nursery for 1 to 2 years before field
planting. Zahidi, Berim and Hayani varieties are known to produce large
numbers of offshoots, while Mektoum and Barhee varieties produce
relatively low numbers of offshoots.
Offshoots are recognised by their curved form
while seedlings have a
straight form. Another way to differentiate between the two is that
seedlings have roots all around their base with no connecting point to
the palm, while an offshoot does not have any roots on the side where
it was connected to the mother plant. Furthermore, an offshoot always
has a mark on one side which is a result of detachment from its parent
palm.
To obtain a high survival rate of transplanted
offshoots, the following
steps are recommended:
Offshoot
selection
The offshoot selected for removal must be
disease and pest free and at
least three to five years old with a base diameter between 20 and 35 cm
(Table 32), weighing over 10 kg but not more than 25 kg because of
handling difficulties. Signs of mature offshoots are the availability
of theirown roots, first fructification and the production of a second
generation of offshoots (Nixon and Carpenter, 1978).
Small offshoots weighing 5 kg and less, if
needed, could also be used,
but their survival potential will be much lower than that of larger
offshoots. They should initially be looked after, for at least two
years, in a nursery, or mist bed in a greenhouse or a shade net
structure (Reuveni et al., 1972). Fungi are usually a serious problem
in a mist bed, and the offshoots must be treated twice a month with a
large spectrum fungicide.
TABLE 32
Relationship between diameter and weight of the
offshoot
| Base
diameter of the offshoot (cm) |
Approximate
weight (Kg) |
| 12 - 15 |
4-8 |
| 15 - 20 |
8 - 15 |
| 25 - 35 |
22 - 35 |
The
best time
for the removal of offshoots and transplanting into the nursery for
rooting (never directly into the field) is after the soil begins to
warm up in the late spring and early summer (September/October in
Southern hemisphere and March/April in the Northern hemisphere).
February/March and September/October are then the most suitable period
for field planting, respectively.
Offshoot
rooting
Two
types of
offshoots occur on a date palm tree: the lower and older ones, and the
upper and younger ones. It is believed that low offshoots are more
active physiologically than high ones; they probably grow faster (the
number of leaves produced increases with age). In fact, the high
offshoots have less carbohydrates than low offshoots, resulting in low
roots production and consequently low survival rate. It is also
suspected that high offshoots develop when no fruit is on the palm.
Early
offshoot
removal is desirable because:
(1) removal
allows easy access to the palm,
(2) removal
improves the development and fruit production of the parent tree, and
(3) planting
young offshoots is advantageous as they will in turn produce a greater
number of offshoots than older ones. |
Numerous
factors to consider when rooting offshoots include: the size of an
offshoot (often expressed in weight), type (upper or lower), origin of
the offshoot, the method of removal and preparation for planting, as
well as treatment of an offshoot after planting (Nixon and Carpenter,
1978).
To
promote
rooting, the base of the offshoot should be in contact with moist soil
for at least twelve months before removal. Production of high offshoots
is primarily of a varietal character but also in some cases related to
a damp climate. For these high offshoots, boxes or plastic bags/Hessian
material could be fastened around the base of the offshoot. Another
technique is to leave them on the mother palm until they mature. They
are then removed and rooted in a nursery (Figure 35a and 35b).
Offshoot pruning
When
the aim is
the production of offshoots, no green leaves should be removed from an
offshoot until it is cut from the mother palm, since the growth of an
offshoot is in proportion to its leaf area. When larger offshoots are
selected for the following year's cutting, all their leaves must be
retained until the offshoots are removed. When leaves interfere with
cultivation, they may be tied together.
When
a date
palm is crowded with offshoots, only 5 to 6 larger offshoots could be
left, considering the tree's equilibrium, and the other smaller ones
could either be totally removed if not needed in the future, or have
their leaves cut back close to the bud to retard their growth.
Offshoots
removal
After
3 to 5
years of attachment to the parent palm, depending on the variety,
offshoots will form their own roots and start producing a second
generation of offshoots. Only at this stage are they ready to be
removed (Nixon, 1966; Nixon and Carpenter, 1978).
Care
and skill,
acquired only by experience, is important in order to cut and remove an
offshoot properly from its mother palm. The operation, usually carried
out by two skilled labourers, starts by irrigation several days before
cutting. Soil is then dug away from the offshoot(s) using a sharp,
straight-blade shovel (a ball of earth, 5 to 8 cm thick, must be left
attached to the roots of the offshoot, with the connection exposed on
each side). Roots should at no time be cut closer than necessary, since
most of the cut roots die and new roots just emerging are susceptible
to injuries (Nixon and Carpenter, 1978).
A
specially
designed chisel is recommended to cut offshoots. It is a rectangular
cutting blade made of tempered steel, which is welded to a solid iron
handle. One side of the blade is fl at and the other bevelled so as to
form a sharp cutting edge. The following chisel dimensions could be
suggested: Blade: 11 cm wide, 22 cm long and 2,5 cm thick; Handle: 120
cm long and 3 cm thick (Figure 36).
Lower
leaves
must be cut off and the remaining ones tied together in order to
facilitate handling. Once the loose fibre and old leaf bases are cut
away and the connection between the offshoot and the mother-palm is
located, the first cut is made to the side of the base of the offshoot
close to the main trunk. The fl at side of the chisel is put towards
the weak point of the offshoot and the bevelled side towards the mother
palm. Injury must be avoided at all times, the offshoot's tender heart
should never be damaged and the cutting operation must be only from one
side to obtain a smooth cut surface.
After
completion of the removal of the offshoot, the old leaf stubs and lower
leaves are cut off close to the fi bre and the basal part left bare of
leaves. Ten or twelve leaves around the bud are retained and tied close
together 6 to 8 cm above the bud with heavy twine or wire. The terminal
parts of these leaves extending beyond the tie (20 cm above the tip -
centre of the offshoot) are also cut off (Figure 37). It is advised
that the cut surfaces of both the offshoot and the mother palm be
covered with a copper sulphate product in order to avoid infection by
Diplodia and other parasites.
Survival of
cut-offshoots depends to a large extent on the variety. Medjool's
offshoot is far more difficult to establish than Deglet Nour or Zahidi.
In
places such
as Fezzan (Libya), some areas of Iraq and Saudi Arabia, and Hadramaout
(Yemen), offshoots are not at all removed and continue to grow outwards
from the original mother palm, producing large clumps consisting of
hundreds of shoots, none of which produces a trunk and of course with
no significant yield (Dowson, 1982).
Planting
offshoots
It
is advisable
that an offshoot never be planted into the field directly after removal
from the mother plant. A rooting period of one to two years in a
nursery is essential in order to ensure an optimum survival rate and to
avoid uneven development of the plantation.
In
most soils,
the early and rapid growth of the offshoot is better when the holes are
prepared one to two months before planting. The size of the hole should
be one m³ and the holes should be filled with a mixture of
topsoil and 10 to 15 kg of manure of high quality (with very little
unmatured matter) and NPK fertilisers. The filled holes should be
irrigated several times to promote the decomposition of the manure and
also to allow the mixed soil to settle in the hole. Well-rotted manure
can be used in holes prepared and irrigated shortly before planting,
but extreme care must be taken to put the manure (and fertilisers) deep
enough to form a layer of soil of at least 15 to 20 cm thick between
the manure and the base of the offshoot.
The
leaf base
of the offshoot should be clearly above the soil level. It is important
to plant the offshoot to the depth of its greatest diameter in order to
avoid the rotting of the base (if it is too low) and to prevent the
water reaching the loose fibre near the bud which causes its
desiccation (if it is too high). The plant water basin, of 1.5 to 1.8 m
in diameter and 20 to 30 cm deep, should be prepared around the
offshoot (Figure 41).
The
soil near
the newly planted offshoots should be kept moist at all times by light
and frequent irrigation. The irrigation frequency is dependent on the
type of soil. Very sandy soils require daily irrigation during the
first summer. Heavy soils require irrigation only once a week; while in
most soils irrigation is required every second or third day. During the
first six weeks (or till the appearance of new growth) the date grower
should always inspect his/her planted offshoots to make sure that the
surface soil does not dry and shrink away from the offshoot. A mulch of
hay or straw around the offshoot will enhance moisture contention, weed
control and finally improve humus in the basin (Figure 38).
Young offshoots
and tissue culture-derived plants should be protected from harsh
climatic conditions (sun and wind during the first summer and cold the
following winter) and against some animals (rabbits, etc.). The use of
shade net/hessian wrapping or a tent of date leaves is recommended
(Figure 39). The top is to be; left open so that new growth may
push
through.
Under
Namibian
conditions (Southern hemisphere), there are two appropriate periods for
planting: February/March and September/October. The first period is
preferable since it allows a longer time for the offshoot to establish
itself before the arrival of the next year's hot summer temperatures,
although it passes through the cold months of winter (June, July and
August) while the plant is still in its initial establishment phase.
The second period (September/October) avoids the cold temperatures and
later receives warm temperatures that allow an active growth followed
by the hot summer (December, January).
To
summarise,
offshoot propagation is true to type but it is not very practical from
a mass propagation point of view, and consequently does not satisfy the
large needs of plant material. The following reasons illustrate this
handicap:
-
Offshoot
production is limited to a certain period in the palm's life span (a
short vegetative phase of about 10 to 15 years);
-
During this
short phase, only a limited number of offshoots are produced (20 to 30
offshoots, at most, depending on the variety);
-
Some
varieties produce more than others (some do not produce offshoots at
all);
-
A mature
specimen with no offshoots will be lost if not propagated through
another technique;
-
Depending on
the care given, a low planting survival rate is frequently obtained
when using offshoots;
-
The use of
offshoots will enhance the spread of date palm diseases and pests;
-
Offshoot
propagation is difficult, laborious, and therefore expensive. |
In
comparison
to the seed propagation technique, offshoots which are axillary
vegetative buds, will offer the following two advantages:
-
The fruits
produced will be of the same quality as the mother palm and ensure
uniformity of produce (true to type).
- The offshoot
will bear fruit earlier than seedlings (by 2-3 years). |
4. Tissue
culture propagation
Palms
are a
much neglected plant group in terms of understanding their development
and vegetative propagation potential. Yet, they are economically
important in tropical and subtropical regions. The rapid propagation of
date palm as well as propagation from a mature specimen, is impossible
due to the limited number of offshoots produced and the fact that
offshoot production is limited to a certain period in the palm's life
span. As mentioned above, seed propagation of date clones and cultivars
is impractical.
The
application
of tissue culture techniques for date palm, also called in vitro
propagation, has many advantages (in comparison to the above two
techniques) and enables the following:
-
Propagation
of healthy selected female cultivars (disease and pest-free), Bayoud
resistant cultivars, or males having superior pollen with useful
metaxenia characteristics which can easily and rapidly be propagated;
-
Large scale
multiplication;
-
No seasonal
effect on plants because they can be multiplied under controlled
conditions in the laboratory throughout the year;
-
Production of
genetically uniform plants;
-
Clones to be
propagated from elite cultivars already in existence, or from the F1
hybrids of previous selections, and seed-only originated palms;
-
Ensure an
easy and fast exchange of plant material between different regions of a
country or between countries without any risk of the spread of diseases
and pests; and
-
Economically
reliable when large production is required. |
The
success of
propagating monocotyledons in vitro has been limited to relatively few
herbaceous species. Similarly, most dicotyledons, successfully tissue
cultured, have also been the herbaceous types. It has been postulated
that in woody plants, the ability to regenerate plantlets using tissue
culture techniques was lower in comparison to herbaceous plants. In
palms, until twenty years ago, little success was achieved in inducing
and maintaining good callus. Plant tissue culture techniques have been
employed to clone a wide range of plants and economically important
palms e.g., coconut, oil and date palms (Cheikh et al., 1989).
In
reviewing
date palm tissue culture, the classification followed will be that of
behaviour and relevant techniques of tissue culture as a whole from a
perspective of their eventual applications to date palm (Zaid and
Djerbi, 1984; Zaid, 1985; 1986a; 1986b).
This
review
also explains the background to the cloning methods applied to the date
palm and explores the wide range of results obtained with embryo
culture, meristematic tissues (shoot tips and buds) and highly
differentiated somatic tissues (leaf, stem, inflorescence and root
sections).
4.1 Embryo
culture
Embryo
culture
involves excising an embryo-aseptically from the seed and planting it
in a sterile nutrient medium (Hoded, 1977). Embryo culture is suggested
to have several potential applications in plant research. It is used to
save embryos that fail to develop naturally in the fruit or seed, or
grow out embryos from interspecific hybridisation where defective
endosperms are common (Johnston and Stern, 1957). Embryo culture may
also be used to reduce lengthy dormancy periods due to physical and/or
chemical inhibitors present in the fruit or seed (Hoded, 1977). Excised
embryos cultured in vitro, free from these inhibitors, usually
germinate immediately. Isolated embryos were also chosen as explant
material in metabolic studies (Raghavan, 1976). The culture of isolated
embryo segments may be useful to study the development of the primary
meristems, organogenesis and the interactions between different organs
(Rabéchault and Gas, 1974). The culture of embryo outside
the seed was first performed with crucifers (Haning, 1904). It has
since become a routine procedure.
With
regard to
date and other palms, callus initiation and embryoid induction was
first observed by Rabéchault (1962) working with oil palm
embryos. Reuveni (1979) reported that callus and roots developed from
the date palm embryo cotyledonary sheath tissue in media containing
naphthalene acetic acid (NAA). This callus continued to proliferate and
to differentiate roots when subcultured if a piece of the cotyledonary
sheath was present. Ammar and Benbadis (1977) established organogenic
callus from date palm cotyledonary sheath of zygotic embryo germinated
in vitro.
Reynolds
and
Murashige (1979) cultured embryo explants of Chamaedores
costaricana Oerst, Howeia forsteriana
Becc., and Phoenix
dactylifera L.
in vitro. Green date palm fruits, harvested two to three months after
pollination were planted in a medium enriched with 2,4-dichlorophenoxy
acetic acid (2,4-D), and a creamy-coloured grainy callus was
subsequently developed. Transfer of this callus to an auxin-free medium
resulted in the development of numerous asexual embryos. Mature zygotic
embryos cultured in nutrient media containing charcoal with high auxins
levels, 10 and 100 mg/1NAA, also produced nodular callus (Tisserat,
1979). Repeated culture resulted in the formation of plantlets.
Tisserat and DeMason (1980), described plantlet formation from date
palm tissue cultures. The morphological development of asexual embryos
from callus closely paralleled excised zygotic embryo germination in
vitro (Figure 40).
Zaid
and
Tisserat (1984) performed a survey study to determine excised embryo
callus production. In the Arecaceae, embryo excised from mature seeds
of 38 species were cultured on modified Murashige and Skoog (MS) medium
containing 3g/L-¹ activated charcoal; with 100 mg
L-¹, 2,4-D and 3 mg L-¹ N6- (2 - isopentyl) adenine
(2-iP). Embryo cultures from 18 of these species produced prolific
callus after repeated reculturing for six months. Zaid (1987) also
cultured embryos of date palm to follow up their development. The
sequence of germination is shown in Figure 41.
4.2
Culture of
date palm meristematic tissues
When
comparing
shoot-tips and lateral buds in vitro versus culturing other explant
sources, the following advantages become apparent:
1. Shoot-tips
and lateral buds are protected by bud scales and leaves, and are
usually easier to surface-sterilise than root or stem explants (Morel,
1960).
2. By
culturing shoot-tips or buds, an entire shoot is already present, thus
only root induction is required to produce a whole plantlet (Morel,
1965; Williams, 1974).
3. The cells
of the shoot-tips and buds are more uniformly diploid than those
derived from less meristematic regions (Murashige, 1975). Presumably,
plantlets derived from naturally meristematic regions are likely to be
clonal and generate faster than other explant sources. |
A
distinction
is made between bud and apical meristem cultures. Lateral bud culture
involves the growth of an entire rudimentary vegetative shoot. Apical
meristem culture, ideally involves only the excision and growth of
apical dome of the shoot usually less than 0.1 mm in diameter and 0.25
mm in length, sometimes with, though preferably without, a few leaf
primordia attached (Cutter, 1965). In contrast to culturing herbaceous
angiosperm shoot apices, few woody angiosperm shoot-tips have been
established in vitro (De Fossard, 1976).
4.2.1 In vitro
culture of date palm shoot-tips
Schroeder
(1970) and Staritsky (1970) employing date and oil palms respectively,
cultured shoot-tips in vitro with some success. However, most of
excised shoot-tips either failed to grow or showed no root
differentiation.
Reuveni et
al.(1972) found that growing tip cultures of date palm responded
irregularly to growth regulators, but optimal leaf development occurred
when media contained 0.1 mg/1 NAA and 0.01 mg/1 kinetin. Callus
occasionally formed at the cut surface of the tip, particularly in dim
light, when low concentrations of auxin and/or cytokinin were present.
Generally this
callus was very short lived and its subculture was unsuccessful
(Reuveni and Kipnis, 1974).
El Hannawy and
Wally (1978) observed some bud differentiation in date palm cultures.
They reported that by adding 200 mg/1 "fermentol" to MS medium
containing 1.0 mg/1 auxin and kinetin, and using an incubation
temperature of 25°C, 60 % bud differentiation occurred. Scharma
et al. (1980), using date palm shoot-tips, reported limited success in
their development due to the browning of the tissue and media. Tisserat
(1979), culturing date palm shoot-tips, found that a high auxin
concentration of 10 and 100 mg/1 NAA and 2,4-D caused a reduction in
the culture weight, and inhibition of shoot growth, but promoted the
formation of yellow-white nodular callus. These nodules were precursors
to asexual embryos. Transfer of callus to nutrient medium containing
lower levels of auxins such a 0.1 mg/1 NAA or 2,4-D allows shoot
development from tips to occur. Male and female shoot-tips were found
to grow equally well. Root initiation was infrequent and did not appear
to be related to the nutrient medium composition.
Zaid and
Tisserat (1983a) cultured date palm shoot tip explants from adult
palms, offshoots, seedlings and asexual plantlets on modified MS
nutrient media containing 10 mg/1 NAA. Differential morphogenetic
responses were obtained dependent on the explant type and parent source
(Table 33). The same authors also determined the action of several
auxins and cytokinins on development of date seedling shoot-tips and
apical meristems (Table 34). Shoot-tip explants consisted of the apical
dome with two to four leaf primordia, and varied in size from 0.5 to 1
mm². Meristems and tips were cultured on modified MS medium
containing 3 mg L-¹ activated charcoal, 0.1-300 mg
L-¹ NAA, 2.4 - D, indoleacetic acid (IAA), indolebutyric acid
(IBA), 4 - chorophenoxyacetic acid and 2iP. Best consistent shoot
regeneration occurred on nutrient media containing 10 mg L-¹
NAA. These shoots were recultured on nutrient media, devoid of
charcoal, containing 10 mg L-¹ NAA or kinetin to obtain
rooting and enhanced shoot development. Best rooting was achieved with
0.1 mg L-¹ NAA with 63% of the shoots initiating adventitious
roots after the first culture passage. Axillary bud outgrowths were
occasionally obtained from shoots cultured on media containing 0.01 and
0.1 mg L-¹NAA only.
TABLE
33
Morphogenesis
obtained from shoot tip cultures derived from various date explant
sources
| Explant
sources (*) |
Survival/
treatment (%) |
Shoot
growth/
culture (%) |
Shoot
length/
culture (%) |
Leaves/
culture |
Rooting/
culture (%) |
| Adult palm |
70 |
85 |
2.12
±.71 |
1.5
±.5 |
0 |
| Juvenille offshoot |
78 |
80 |
2.75
±.69 |
2.5
±.6 |
0 |
| Seedling |
85 |
100 |
2.35
±.65 |
2.0
± 0.0 |
60 |
| Asexual
plantlet |
95 |
100 |
1.67
±.39 |
2.2
±.4 |
80 |
(*)
15-20
cultures employed per treatment; results taken 8 weeks after planting.
TABLE
34
Influence of
growth regulators on the growth of date palm shoot tips
| st
levels (mg/l) |
Shoot growth (%)
Growth Regulator Type |
|
NAA |
2,4-D |
IAA |
Kinetin |
BA |
2iP |
| 0.0 |
58 |
50 |
42 |
80 |
58 |
80 |
| 0.1 |
58 |
- |
- |
60 |
67 |
60 |
| 0.3 |
67 |
47 |
33 |
40 |
58 |
40 |
| 1.0 |
58 |
53 |
42 |
53 |
50 |
53 |
| 3.0 |
67 |
53 |
33 |
66 |
58 |
66 |
| 10.0 |
75 |
53 |
33 |
73 |
33 |
73 |
| 30.0 |
- |
67 |
50 |
- |
33 |
- |
| 100.0 |
75 |
13 |
- |
60 |
42 |
60 |
| 300.0 |
58 |
6 |
25 |
53 |
42 |
53 |
4.2.2
In vitro
culture of date palm buds
Most
buds of
date palm were reported to die within the first 30-50 days after
planting in vitro (Reuveni and Kipnis, 1972; Schroeder, 1970). Only the
largest and most distinctly differentiated buds grew. These buds
exhibited leaf expansion and produced additional leaves. Tisserat
(1979) and Zaid (1981) also investigated the conditions for bud
development, and found that in nutrient medium shoot-tips and lateral
buds grew equally well on the same medium. Callus cultures have been
initiated from axillary buds of 2 to 4 year old date palm offshoots.
Zaid and Tisserat (1983b) found that subcultured lateral buds callus on
nutrient media devoid of charcoal and supplemented with 0.1 mg/1 NAA,
produced adventitious plantlets. Tisserat and DeMason (1980) found that
on a medium devoid of 2,4-D and 2-iP, sectioned buds callus consisted
of two distinct types of tissues; a loose friable tissue and compact
aggregates. The friable portion of the callus was composed of large
non-meristematic cells and disorganised clumps which were highly
vaculated and ranged in diameter from 20-40 µm. This tissue
was not involved in embryo formation and was generally found
surrounding the aggregate clumps which consisted of densely cytoplamic
cells containing few vacuoles and usually were 8-20 µm in
diameter. The formation of vascular bundles within the asexual plantlet
at the 8- week old stage corresponded to that found in the zygotic
seedling.
Starting
from
the bottom of young leaves, soft tissues, shoot tips or axillary buds
of date palm offshoots (Figure 42),and using MS half strength or
Beauchesne medium supplemented by various auxins at a low
concentration, buds were obtained after six months of in vitro culture
(Beauchesne et al., 1986) (Figure 43).
Early rooting
of date palm tissues reduce bud multiplication and is, occasionally,
responsible for the inhibition of bud initiation. In order to solve
this problem, the bottom of young leaves of date palm offshoots were
cultured on eight different nutrient media with different levels of
growth regulators (Anjarne and Zaid, 1993). High level of auxins,
especially NAA, allowed root initiation. These roots showed a rapid
growth after subculturing on a medium containing a lower level of
auxins. Furthermore, organogenesis was inhibited on media with a low
concentration of auxins.
Vitrification
phenomenon of date palm tissues is a handicap for the successful in
vitro multiplication of some date varieties and selected clones. In
order to overcome such a problem, four culture media with different
ammonium/total nitrogen ratio were tested, and bottom young leaves from
offshoots of AGUELLID variety were used (Bougerfaoui and Zaid, 1993).
It was found that ammonium plays an important role in the vitrification
process. High levels of ammonium nitrate were found to enhance rapid
growth and consequently tissue vitrification (46 to 53 % of cultures);
while this phenomenon is reduced to 14 - 19 % in media with low levels
of ammonium nitrate.
4.3 Culture of
highly differentiated date somatic tissues in vitro
4.3.1 Leaf
cultures
Callus
developed from a seedling date palm leaf (Schroeder, 1970), and gave
rise to roots several months later. Similar results were obtained by
Reuveni and Kipnis (1974). In their study, primordial leaves survived
in culture and expanded, especially in the presence of light. The
addition of plant growth regulators at concentrations of 0.1 mg/l and
above was injurious to cultured leaves.
Eeuwens
and
Blake (1977) working with date palm leaf found development of root
initials to be enhanced by the presence of a low level of gas and
auxins, and by a reduction in either the level of minerals or sucrose.
Phoenix leaf petiol explant has initiated roots within 6 weeks when
subcultured onto a medium with high levels of auxin (Eeuwens, 1978).
Root initiation was not prevented by the presence of high cytokinin or
low sucrose levels, but occurred more frequently in media containing
high sucrose and reduced cytokinin levels. Poulain et al (1979)
obtained some callus at the base of young date palm leaves. Buds
developed at the insertion zone between young leaves and rachis. Roots
were obtained on MS supplemented with a combination of low auxin levels
such as 1.2 and 3 mg/l NAA, IBA, and IAA, respectively. Scharma et al
(1980) noted callus from leaf petioles of date palm initiated in media
employed by Staritsky (1970) or using Eeuwens Y/3 mineral formulation
(Eeuwens, 1976).
Zaid
(1981),
working with date palm leaf explants from adult trees, offshoots,
seedlings and asexual plantlets, found that only subcultured leaf
callus from seedling and asexual plantlets produced roots.
4.3.2
Stem
culture
Staritsky
(1970) and Smith and Thomas (1973), both working with oil palms, and
Eeuwens (1978) with coconut and date palms, obtained a white callus on
a few stem cultures. Further attempts to subculture this callus failed.
Phoenix stem
explants reportedly enlarged considerably in size during the first few
weeks of culture (Tisserat, 1979). Repeated culture to fresh media
resulted in the formation of non-friable nodular callus. Plantlets were
developed from this callus. Poulain et al. (1979) working with date
palm stem tissues also successfully initiated callus.
4.3.3
Inflorescence culture
Inflorescences
of several species have been cultured in vitro (Nitsh, 1963). Since
1973, several workers attempted to culture palm inflorescences.
Explants of female and male oil palm inflorescences were cultured on a
variety of media and usually developed somewhat normally, but callus
was not obtained (Smith and Thomas, 1973). A high auxin level was
speculated to be necessary to disrupt normal development. This has
subsequently been confirmed in date palm (Eeuwens and Blake, 1977).
Date
palm
ovules, carpel tissue, parthenogenetic endosperm, and the fruit stalk
blackened within 24 hours after culturing on nutrient media, and
subsequently died (Reuveni and Kipnis, 1974). Also cultures of date
palm floral bud reproductive tissues and especially male anthers,
usually turned brown and died after a few weeks in culture (Tisserat et
al., 1974). De Mason and Tisserat (1980) found that in vitro
applications of auxins to media increase the frequency of visible
expanded carpels developing from supposedly date palm male fl owers.
Vestigial
female date carpels on surviving male flowers enlarged and became quite
prominent (Tisserat, 1979). White friable callus usually initiated from
the floral bud strand (Tisseral et al., 1979). In some cases, roots and
embryoids were initiated from explants of Cocos inflorescences
rachillae (Eeuwens, 1978) and from date palm (Tisserat, 1979). Roots
have not been initiated on inflorescence rachis explants which lack
leaf or meristem tissue.
Date
palm
inflorescence culture was also largely investigated by Drira (1981).
Morphogenetic responses were found dependent on the origin and
physiological stage of the explant.
4.3.4
Root
culture
Staristsky
(1970) and Schroder (1970) were the first to investigate root cultures
in palms in vitro. Oil palm root and root primordia failed to develop.
Schroder (1970) observed that date palm root pieces in turn developed
secondary rootlets but did not produce shoots. Eeuwens (1978) found
that isolated roots excised from cultured explants of date and coconut
palms continue growth and produce laterals when subcultured on liquid
static media. Callus was also reported to form at the root tip region
of young date palm seedlings (Smith, 1975; Smith and Thomas, 1973).
This callus had produced leaves and shoots. Other investigators
(Scharma et al., 1980) reported no growth for cultured date palm roots.
Usually, severe browning and death of root explants occurred within the
first few weeks of culture. However, Zaid and Tisserat (1983a; 1983b),
obtained some callus from seedlings and asexual plantlets roots when
callus failed to exhibit any morphogenic response.
4.4 Browning of
tissues and media in date palm tissue culture
During
the
course of in vitro growth and development, plant tissues not only
deplete the nutrients that are furnished in the medium, but also
release substances that can accumulate in the cultures. These
substances, such as phenols, may have profound physiological effects on
the cultured tissues. Date palm tissue cultures, like those of many
other plants, have been commonly observed to release discolouring
substances into the medium which inhibit their own growth. For date,
injury through cutting of tissue is accompanied by secretion of the
substance(s) into the medium. The intact organ, as exemplified by
embryos or whole leaves on tips do not brown and thus grow well in
culture (Reuveni and Kipnis, 1974). Browning of the tissue and the
adjacent medium is assumed to be due to the oxidation of polyphenols
and formation of guinones which are toxic to the tissues (Maier and
Metzlier, 1965; Zaid, 1987).
To
minimise
browning, Murashige (1974) has suggested the pre-soaking of explants in
ascorbic and citric acid solutions and adding them to the culture
medium. Zaid and Tisserat (1983a; 1983b) soaked their date palm
explants in an anti-oxidant solution (150 mg/l citric acid and 100 mg/l
ascorbic acid) prior to the surface sterilisation treatments. Addition
of a combination of adsorbents including citrate, adenine and
glutamine, retarded browning in date palm explants (Rhiss et al.,
1979).
Addition
of
other adsorbents to nutrient media, such as dihydroxynaphtalene,
dimethylsulfoxide, were ineffective against browning in date palm
explants (Zaid, 1984). Apavatjrut and Blake (1977) suggested that
browning could be eliminated by a nutritionally balanced medium.
Excision of browning explant parts during culture was also advocated to
prevent this problem (Zaid, 1984).
The
use of
charcoal is preferred over cysteine and other adsorbents because the
latter are often toxic to the plant tissues at higher concentrations
(Zaid, 1984, 1990). Addition of 3 % charcoal has caused substantial
root and shoot growth of date embryos. Constantin et al. (1977)
suggested that the growth regulators required for callus growth and
shoot development for tobacco are adsorbed by charcoal addition.
Similarly, Fridborg and Erikson (1975), postulated that the addition of
charcoal to a culture medium drastically alters the properties of the
medium. Hence, growth regulator substances are tested at high levels
(e.g. 10 and 100 mg/l) with charcoal included in the nutrient media to
obtain beneficial effects on tissues (Zaid, 1990; Zaid et al., 1989).
4.5
Cryopreservation of date palm shoot tips
Studies
on the
cryopreservation of date palm for germplasm collections were initiated
by Towill et al., (1989). Shoot-tips were excised from 2 month-old
seedlings derived from the cultivar "Medjool", precultured for 2 days
and then cooled to liquid nitrogen (LN) temperatures using procedures
described for potato and mint species. Viability of treated shoot-tips
was assessed by growth in vitro. Dimethylsulfoxide (DMSO) in
concentrations up to 10 % was not toxic, although growth was slower
than untreated shoot-tips. Several combinations of DMSO and sucrose
were effective in obtaining survival after LN exposure. In most cases,
the LN-treated shoot tips developed directly into a shoot without
callus formation (Towill et al., 1989).
4.6
Organogenesis and asexual embryogenesis
Date
palm
plantlets may be produced through either; asexual embryogenesis, i.e.
initiation and germination of somatic embryos from callus; or
organogenesis, i.e. rooting and division of shoot tips and lateral
buds.
Organogenesis
technique, based on meristematic tissues potentiality, avoids callus
formation and does not use 2,4-D. Growth substances included in the
media are used as low as possible.
Organogenesis
technique consists of 4 steps: Initiation of meristematic buds (also
called the starting step), multiplication (Figure 42), elongation and
rooting (+ swelling step). The success of such a technique is
tremendously dependent on the success of the first step (initiation);
Furthermore, various problems met at other levels have their origin at
the initiation phase. These technical problems could be summarised as
follows:
*
At
the
initiation phase
- Physiological
stage of the offshoot, weight, age, signifi cation degree, period of
introduction.
- Initiation:
too long.
- Bacterial
contamination.
- Browning
phenomenon.
- Varietal
response to the technique/Lack of reactions of some clones and
varieties.
- Yield of the
technique/offshoot.
- Precocious
root development.
- Lack of
results repetition.
*
At
the
multiplication phase
- Low and
irregular multiplication rate.
- Decrease of
regeneration capacity (precocious rooting).
- Loss of
totipotency for some varieties.
* Rooting and
elongation
- Low efficient
rooting.
*
At
the
acclimatisation phase
- Low rate of
survival. |
Asexual
(also
called somatic) embryogenesis, is based on the callus production and
multiplication, followed by the germination and elongation of somatic
embryos. Up to now, this technique had shown to be genotype independent
with a high rate of multiplication and a high survival rate upon
transfer to soil.
4.7
True-to-Typeness
There
is always
a dispute amongst date growers, technicians and scientists about the
true-to-typeness of plants produced in vitro. It is worth mentioning
that tissue culture-derived plants of many species are subject to
somaclonal variation in particular, and to genetic variations in
general. Unlike epigenetic variations, which are at physiological level
with non-heritable effect, genetic variations are affecting the genome
and consequently are heritable (Pierik, 1987; Zaid, 1987; 1990).
According
to
these authors, factors causing variations in plant tissue culture are:
-
Technique
used for propagation;
- Nature of
plant mother (chimera);
- Type of
growth regulators used;
- Type of
explant used (ploidy gradients: apex to root);
- Age of
culture (> one year);
- Medium
composition; and
- Incubation
conditions. |
Most
of the
commercial laboratories are doing their best to ensure the true to
typeness of the produced date plant material. Various techniques are
used to produce and certify the conformity of the plants
(Histo-cytology:Figure 44), iso-enzyme, RFLP (Figure 45), RAPD
techniques). In most cases, finger printing is the technique actually
used, but according to our experience we feel that the field response
is the only reliable way to confirm if the palms derived from tissue
culture are true to type to the plant mother.
Up
to now, only
two cases of variation with Medjool and Barhee have come to our
attention. Out of 2000 Barhee palms derived from asexual embryogenesis,
only 2 are showing an abnormal vegetative growth (a ration of 0.1 %).
These palms are marked and their fruits will be compared to the mother
variety (Figures 46, 47 and 48).
4.8 Commercial
production
Various
laboratories in the world have made attempts to propagate date palm by
tissue culture techniques. According to the knowledge of the authors,
success has been achieved at only a few international laboratories
(Table 35).
Some of these
laboratories are recent (2 to 3 years), while others have been
functioning for approximately 15 years. There are 9 functional
laboratories known to the authors. These are found in England (1),
France (2), Israel (1), Morocco (1), Namibia (1), UAE (1), Oman (1),
and India (1). Information about the last two laboratories is not
available.
The commercial
laboratory of the "Domaine Agricole El Bassatine" (Morocco), which
since its start had produced ± 500,000 plants, is reserving
all its production for national use. No signifi cant sale outside
Morocco has been implemented because all the production is destined to
rehabilitate the Moroccan Date plantations destroyed by the Bayoud
disease.
The remaining
laboratories (England, France, Israel and Namibia) are potential
sources of date plant material. Most of these laboratories' efforts
were focused on the Medjool (and Barhee recently) variety with an
average sale price (FOB) of about 20 to 23 US$ per plant. Delivered
plants have only juvenile leaves and still need to be hardened-off by
the buyer before fi eld planting (Figure 49). Note that the selling
price depends on the variety, the number of plants ordered and the
growth stage at delivery.
TABLE
35
List of
international date palm commercial laboratories(*)
| Country |
Company |
Address |
| UNITED KINGDOM |
- DATE PALM DEVELOPMENTS |
Baltonsborough, Somerset
BA6. 8QG, United Kingdom
Tel: (+44) 1458 850576
Fax: (+44) 1458 851104 |
| France |
- MARIONNET G.F.A. |
21 Rue de Courmemin 41230 Soings - France
Tel: (+33) 254 987 103
Fax: (+33) 254 987 523 |
|
- PALMDAT - France |
"Laboratoire de Physiologie
Végétale"
"Recherche et Développement"
Marolles 37460, Genille,
France
Tel: (+33) 247 5952 52
Fax: (+33) 247 59 59 18 |
| ISRAEL |
- RAHAN MERISTEM |
Propagation Nurseries
Kibbutz Rosh Hanikra
Western Galilee 22825, Israel
Tel: (+972) 4 985 7100
Fax: (+972) 4 982 4333 |
| MOROCCO |
- DOMAINE AGRICOLE EL BASSATINE |
B.P. 299 Meknes, Morocco
Tel: (+212) 5 50 0493
Fax: (+212) 5 50 0730 |
| NAMIBIA |
- PALMDAT NAMIBIA |
P.O. Box 20519
Windhoek, Namibia
Tel: (+26461) 230480
Fax: (+26461) 250889 |
| UNITED ARAB EMIRATES |
UNITED ARAB EMIRATES UNIVERSITY - DATE PALM
DEVELOPMENT RESEARCHUNIT |
P.O. Box 81908-Al-Ain
Tel: (+9713) 8732334
Fax: (+9713) 7832472 |
| Others in Middle East (Oman)
and in India |
- No information available |
|
| Total Laboratories |
9 |
|
(*)
There is no
order of importance in the list, which should also not be considered as
exhaustive. Countries were classified in an alphabetical order.
5.
Acclimatisation and hardening-off of tissue culture-produced date
plants
5.1 Introduction
Although
in
vitro mass plant propagation has become commercially feasible, many
problems hinder its application to economically important crops.
One
of the
major obstacles concerning the practical application of plant tissue
culture to mass propagation has been the
difficulty of
successful transfer of plantlets from in vitro conditions to a soil
medium. Losses from 50 to 90 % of in vitro propagated plantlets of many
species have been encountered at the time of transfer to soil (Zaid and
Hughes, 1989a; 1989b). This is unfortunate because the ultimate success
of plant tissue culture as a commercial means of plant propagation
depends on the ability to transfer plantlets out of culture, on a large
scale, at low cost and with a high survival rate.
It
is
appropriate at this level to differentiate between the acclimatisation
of date palm vitro plants at the laboratory's glasshouse and their
hardening-off at the farmer's nursery.
5.2
Acclimatisation
Acclimatisation
presents challenges at least equal to those posed by the initiation of
cultures because it marks the end of artificial control and the
beginning of autonomous plant growth. Approximately 20 years ago it was
stated that research concerning the preparation of in vitro plantlets
for transfer to soil had been neglected (Murashige, 1974). Since that
time many scientists have become interested in the effects that the
transfer process has on tissue cultured plantlets (Zaid and Hughes,
1995a; 1995b).
The
culture of
date tissue in vitro with almost 100 % relative humidity within the
culture vessel can lead to various abnormalities in the plant structure
(Zaid and Hughes, 1989c). Plants of many species produced in vitro
often show morphological, structural, physiological and biochemical
differences from those produced conventionally. These include reduced
epicuticular wax deposits (Figure 50), altered leaf anatomy (Figure
51), excessive water loss and stomatal abnormalities compared to
greenhouse grown plants (Zaid, 1995; Zaid and Hughes, 1995c).
It is worth
mentioning that loss of viability is attributed to poor control of
water loss from the date plants and their heterotrophic nature.
Stomatal
development and frequency can be affected by water availability, light
intensity, temperature, humidity and osmotic concentration of the
culture medium (Zaid and Hughes, 1995b).
Even when
gradual hardening off has been used, poor survival and slow growth of
date plantlets have commonly been reported. Such a low survival rate
(that sometimes reaches below 50 %) is caused by several factors which
are mainly young physiological stage of plantlets to transfer,
inadequate root system, unsatisfactory irrigation schedule, and lack of
technical care at the in vitro laboratory stage.
Several
techniques have been used to acclimatise date plantlets and improve
their survival during establishment under greenhouse conditions. The
effectiveness of these methods depended upon ambient conditions, and
most methods have involved environmental modifications. Mentioned below
are the three most important factors to be taken into account by the
manager of a date palm propagation laboratory in order to ensure a high
survival rate and fast growing situation of date palm tissue
culture-derived plantlets:
5.2.1
Physiological stage
Date
palm
plantlets are ready for transplanting only when they gain the following
characteristics:
-
Two to three
healthy and enlarged leaves with no curling phenomenon;
- A shoot
length of at least 10 to 15 cm from stem base to the highest point of
the leaves;
- A shoot base
with an onion bulb-like form (also called pear-shaped crown);
- A well
developed root system with an average of 5 cm in length. Adventitious
rooting is obtained by trimming the primary roots to 1 - 1.5cm in
length and reculturing the plant to an agar nutrient medium containing
0.01/0.1 mg/l NAA without charcoal; and
- Well
acclimatised plant as a final product (Figure 52). |
Plants
are then
rinsed in distilled water to remove adhering agar and residual sucrose.
A spray with Benlate solution at 0.5 % (or any wide spectrum fungicide)
is important since it protects the plant from fungal attack.
In
order to
achieve the above, and consequently produce a well pre-acclimatised
date plant that will survive the transplanting stress, it is
recommended that the following be ensured:
-
Do not
transplant any plant until it gains the previously mentioned
characteristics;
- Enhance a
root-elongation process by using auxins at the last in vitro stage;
- Increase the
light intensity during the last 4 to 6 weeks; and
- Create an
artifi cial osmotic stress (at the nutrient medium level). |
5.2.2
Transplanting to soil medium
The
transplanting operation should be done as quickly as possible to avoid
plant dehydration and avoid root damage as far as possible. The soil
medium must always be sterile and usually consisting of 1 peat: 1
vermiculite (v/v) mixture. Sterile sand with a large grain size could
also be added to improve drainage. Bark is to be avoided because it
dries out rapidly and causes a water stress situation. To summarise,
the substrate should be a well drained one, yet with good water
retention capacity. The adequate pH to work with should be about 6.5.
Plastic
pots
(7.5 - 12.5 cm), jiffy peat pots or trays (25 plants; in case of
commercial production) are often used for date palm transplanting.
5.2.3
Environmental conditions
Plants
are
immediately irrigated with 50 % Hoagland's solution or 10 % MS solution
before their incubation into a micro tunnel located in an
environmentally controlled glasshouse (or a large plastic tunnel).
These
environmental conditions will ensure a high relative humidity (90 - 95
%) and a constant temperature ± 25 - 26°C day time
and 21 - 22°C during the night. Bottom heating of the micro
tunnel (± 23°C) was found to be very helpful.
To
ensure a
high survival rate, date palm tissue culture-derived plants should be
adapted to gradually decreasing humidity and gradually increasing
light. The light intensity is important during the first 3 to 4 weeks
in the glasshouse (around 10,000 lux) with a 16 hr photo period.
Benlate is to be applied to the foliage once a week, and irrigation
using 10% MS solution (or 50 % Hoagland) every 3rd or 4th day depending
on the hygrometry level of the micro tunnel.
Four
to six
weeks later, the plastic of the micro tunnel is gradually opened in
order to decrease humidity and prepare the plants to adapt to the large
glasshouse (or tunnel) conditions which preferably should have a fog
system. Plantlets are now ready to be transplanted to larger plastic
bags.
It
is worth
mentioning that at all stages, water should never be sprayed form the
top of the plant. Plants could stay in the glass house (or a tunnel)
for a period between 3 to 4 months before their transfer to a less
environmentally controlled nursery, which is usually at the farmer's
level, for their further hardening-off process.
5.3
Hardening-off
Plantlets
received from a laboratory are usually about 35 to 45 cm long with 4 to
5 leaves among which are 0 to 2 pinnae leaves (called also permanent
leaves). The plant must have a thick shoot system and the base must be
similar instate to that of a large onion bulb (pear-shaped). As stated
above, the plant must have a well developed root system.
Transportation
of these plants must be realised in a proper manner and plants must
preferably not be stacked on top of each other to avoid stem breakage
and/or leaf damage. Transport must preferably also be in one stage and
if plants/truck are to stay over somewhere, it must be in a shaded
area; watering should not be neglected if transport takes up several
days.
It
is
recommended that, upon reception of this material by the date grower,
plants are transferred to larger bags (7 to 10 litres capacity) with an
adequate substrate, usually sand (soil), vermiculite and gravel at a
ratio of 1:1:1, respectively. Transplanting should be done properly
with no disturbance to the root system. Original substrate around the
roots should stay intact. Plants are then left in the nursery for
approximately 8 to 12 months depending on surrounding conditions and
care given, till most of them reach the 4 pinnae leaf stage. The date
grower is advised to co-ordinate the purchasing and the hardening-off
period, to ensure that planting can betimely implemented (during
February/March for Southern hemisphere and September/October for
Northern Hemisphere).
The
nursery
size and type are related to the number of plants to be hardened-off.
An average size of 150 m² will be adequate for 1,000 plants.
An ultra-violet resistant shade net of 80 % is recommended during the
first 6 months (Figure 53). During the summer time, the top of the
nursery should have a double layer of the shade net for insulation
purposes. The nursery should be well located (close to several trees to
benefi t from their shade) but also in a protected area to avoid sand
storms and severe wind. A water tap should be installed inside the unit
for easy irrigation and the unit must be enclosed to avoid animals
getting in and eating the plants.
Irrigation
is
an important factor and must be implemented once a week in winter time
and at least twice a week during summer. Water should never be sprayed
on top of the plant; soil is to be mounted around the base of the plant
so water can not get into its heart.
Fertilisation
is to be applied once per month: apply 5 g of ammonium sulphate/plant
bag (5 % nutrient solution; thus 15 kg deluded per 63 litres water for
650 plants). Apply 120 ml of solution per plant bag.
Control
of
diseases and pests is also recommended and the use of Benlate (or any
other large spectrum fungicide) has proven to be highly efficient.
Foliar spray of Benlate is to be applied every 3 to 4 weeks.
Close
monitoring is advised as mistakes could be disastrous; It is from our
own experience, that we recommend a close follow-up by the date grower.
If all above recommendations and advice are respected, the date grower
could expect a survival rate between 90 and 95 % (Figure 54).
In
Namibia, a
total of 10,007 plants of various date palm varieties were hardened
during 1996 and 1997 in both Naute and Eersbegin project sites (Table
No. 36).
The
results
obtained are satisfactory and after 8 to 12 months (depending on the
variety and the source), a final survival rate of 92% was obtained
(9,177 plants survived and successfully passed the hardening-off
operation out of 10,007 plants).
TABLE
36
Hardening-off
of date palm tissue culture plant lets: Survival rate (16/06/1997)
| VARIETIES |
ORIGINAL NUMBER OF
PLANTS
ORDERED |
ORIGIN OF PLANT MATERIAL |
TOTAL SURVIVAL |
RATE OF SURVIVAL
(%) |
| Medjool du Roi |
2,922 |
RSA |
2,723 |
93.10 |
| Medjool Marionnet |
2,650 |
France |
2,411 |
90.90 |
| Kush Zabad |
120 |
UK |
106 |
88.30 |
| Khalas |
90 |
UK |
84 |
93.30 |
| Hilali |
90 |
UK |
88 |
97.70 |
| Nabutsaif |
120 |
UK |
116 |
96.60 |
| Khenezi |
135 |
UK |
35 |
25.90 |
| Barhee |
1,965 |
UK |
1,854 |
94.30 |
| Bou Feggouss |
1,225 |
France |
1,225 |
100 |
| Deglet Nour |
120 |
France |
117 |
97.50 |
| Khadrawy |
45 |
France |
01 |
02.20 |
| Anbara |
50 |
UK |
35 |
70.00 |
| Sukkari |
175 |
UK |
162 |
92.50 |
| Khissab |
90 |
UK |
87 |
96.60 |
| AbuNaringa |
120 |
UK |
105 |
87.70 |
| Lulu |
90 |
France |
28 |
31.10 |
| Total |
10,007 |
|
9,177 |
91.70 |
- Immediately
after transplanting, an average percentage a loss of 3.2% occurred.
- After 8 to 12
months in the nursery, the final survival rate was about 92% (9,177 out
of 10,007 plants). |
(Source: Date
Production Support Programme in Namibia; FAO-UTF/NAM/004/NAM; 1997)
Figure 34.
Date palm
seedling plantation to select salt tolerant clones at Guanikontes
(Swako-pmund, Namibia)

Figure 35
Rooting of
off-shoots:
A - Normal
axillary offshoots after their separation from the mother palm.

B - High
offshoots on the palm using plastic bags filled with saw-dust.

Figure 36.
Various types
of chisel used around the world
A -
American
type B - normal and most common C - traditional type
(Source:
Munier, 1973).
Figure 37.
Offshoots
pruning methods:
A -
as an
onion-bulb B - average pruning C - short pruning
Figure 38.
Basin around a
young date palm (1.5 to 1.8 m diameter and 20 to 30 cm deep) with wheat
straw as a mulching.

Figure 39.
After planting
protection against harsh climatic conditions:
A - Use of
hessian wrap for offshoots

B - Protection
unit made of wire and shade net for tissue culture plants

C - Protection
tent made of date leaves.

Figure 40.
Comparison of
asexual embryo (right) with excised zygotic embryo (left) at the
cotylegon elongation stage

Figure 41.
Sequence of
germination for Phoenix
dactylifera
cultivar Sayer excised embryos cultured on a modifi ed Murashige and
Skoog medium containing 0.3 activated charcoal.

From left to
right: early cotyledon elongation stage (1 week old); emergence of
first foliar leaf (3 week old); and established seedling in vitro (6
week old).
Note that the
cotyledon haustorium is much reduced in size in all stages of seedling
develop-
|
Figure 42.
Various types
of date palm explants used in organogenesis technique (mostly the
bottom of young meristematic leaves)
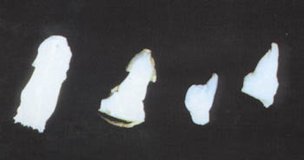
Figure 43.
Multiple shoot
formation of date palm "Tademant" variety
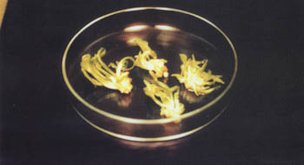
Figure 44.
Cross section
of date palm shoot tip
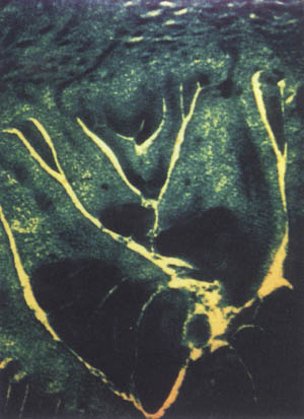
Figure 45.
Zymogram of
date palm "Black Bousthami" variety

Figure 46.
Medjool palm
derived from asexual embryogenesis showing abnormalities; It looks like Black Scorch attack
(Eden Expt.
Station, Israel, 1996)

Figure 47.
Barhee palm
derived from asexual embryogenesis showing morphological abnormality
(Ref. G12-Block2, Naute project, Namibia)

Figure 48.
Large leaf
size
as an abnormality (Right: Variant Barhee; left: normal Bar-hee leaf)
(Ref B14-Block 2, Naute Project, Namibia).

Figure 49.
Boufegouss
variety plants after hardening at the laboratory's glasshouse

Figure 50.
Comparison of
leaf epicuticular wax between greenhouse- grown, tissue culture-
derived (Polyethylene glycol-treated and non treated plants); Note five
varieties were tested.
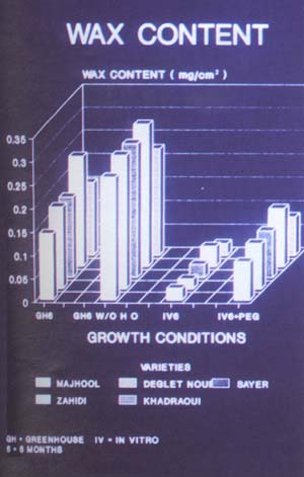
Figure 51.
Leaf anatomy of
a Med-jool date palm. Note the size of the bulliform cells.
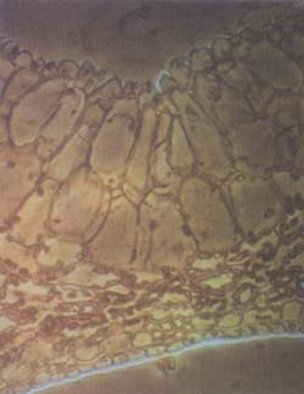
Figure 52.
Well
acclimatized plants ready to go through the hardening-off process.

Figure 53.
An ultra violet
resistant shade net of 80% is commonly used for date palm nursery
(hardening-off
at the date grower's level).

Figure 54.
Various stages
of growth and development of date palm tissue culture plants during the
hardening-off process.

From right to
left: 3 months, 6, 9 and 12 months old.
Back to
Date Palm Page
|